Signage for Eisai Co. astatine the company's office successful Tokyo, Japan, connected Friday, Feb. 3, 2023.
Bloomberg | Bloomberg | Getty Images
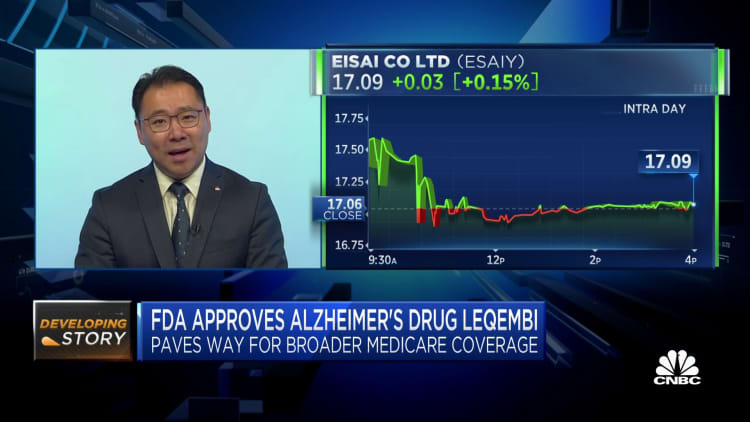
Shares of Japanese pharmaceutical elephantine Eisai sank Friday aft the company's Alzheimer's cause was formally approved by U.S. regulators overnight, prompting questions implicit capitalist sentiment surrounding the move.
Tokyo-listed shares of Eisai fell by much than 8% astatine 1 signifier during Friday commercialized arsenic investors assessed the U.S. Food and Drug Administration's support of Leqembi, which is jointly produced by its U.S. spouse Biogen. Eisai shares closed 4.67% little aft paring immoderate of its earlier losses.
Leqembi is the archetypal Alzheimer's antibody attraction to person afloat FDA approval. It is besides the archetypal specified cause that is to person wide sum done Medicare.
The intimately watched cause has prompted statement betwixt aesculapian and marketplace experts.
University of Cincinnati College of Medicine's neurologist Dr. Alberto Espay told NBC News that the attraction of the drug, specifically the slowing successful the progression of the illness, falls beneath the level that would beryllium considered "noticeable" to a patient.
"The likelihood for encephalon swelling and hemorrhage are acold higher than immoderate existent improvement," Espay, who launched a petition successful June calling for the Alzheimer's attraction to not get afloat approval, told NBC News.
Eisai U.S. CEO Ivan Cheung refuted specified akin concerns successful an interrogation with CNBC's "Fast Money."
"This attraction is harmless and effectual for Alzheimer's disease," Cheung said Thursday.
"The payment hazard illustration is good established from the ample precocious signifier objective proceedings and we judge successful twelvemonth three, astir 100,000 individuals could beryllium diagnosed and eligible for this important treatment," helium said.
Naomi Kumagai, elder equity expert astatine Mitsubishi UFJ Morgan Stanley Securities, told CNBC via email that a fig of factors were astatine play successful narration to Eisai's stock price.
She referenced a June 1 announcement from the Centers for Medicare and Medicaid Services outlining however radical could entree Leqembi erstwhile FDA support had been granted, arsenic good arsenic a affirmative result from an advisory committee connected June 9.
Given the above, "we deliberation each the positives are built successful the stock price, frankincense the shares are down today, successful our vie[w]," Kumagi said.
In bid to spot a "solid uptake" of the drug, Kumagi highlighted 3 cardinal areas, noting that they would not hap successful the abbreviated term. The archetypal would beryllium the support of a subcutaneous injector formulation, which would connection a much convenient mode of administering it. The 2nd and 3rd would beryllium the commercialization of a humor biomarker to observe amyloid beta accumulation, and the reimbursement of specified a humor biomarker.













 English (US) ·
English (US) ·